
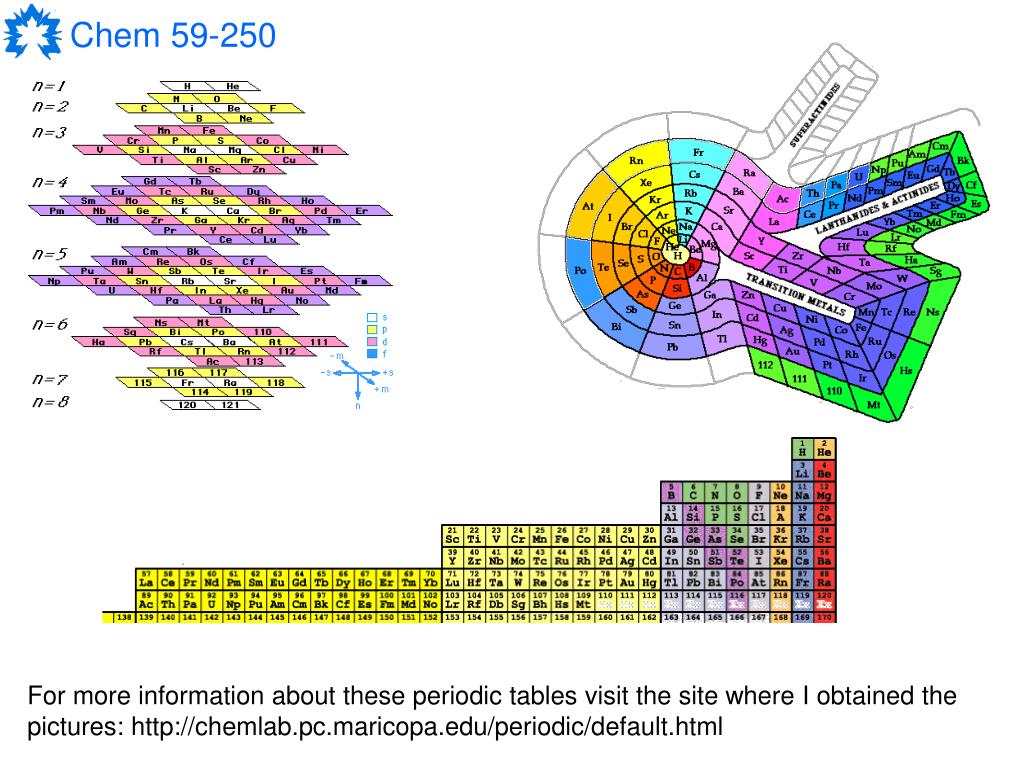
The IUPAC system, on the other hand, offers the obvious convenience of providing a number for each column.
#Www chemlab pc maricopa edu periodic periodic html series#
The North American system assigns no group numbers to these, or to the two rows set aside at the bottom, representing the lanthanide and actinide series of transition metals. The "dip," which spans 10 columns in periods 4 through 7, is the region in which the transition metals are listed. Group 1 in this system consists of hydrogen and the alkali metals Group 2, the alkaline earth metals groups 3 through 6, an assortment of metals, nonmetals, and metalloids Group 7, halogens and Group 8, noble gases. The groups numbered in the North American system are the two "tall" columns on the left side of the "dip" in the chart, as well as the six "tall" columns to the right of it. Both versions of the periodic table show seven periods. In particular, the North American system numbers only eight groups, leaving 10 columns unnumbered, whereas the other system -approved by the International Union of Pure and Applied Chemistry (IUPAC) -numbers all 18 columns. The periodic table is examined in depth within the essay devoted to that subject, and among the specifics discussed in that essay are the differing systems used for periodic-table charts in North America and the rest of the world. For one thing, it makes it possible to see at a glance families of elements, many of which either belong to the same group (column) or the same period (row) on the table. Certainly other organizational systems exist, but Mendeleev's table is the most widely used -and with good reason. HOW IT WORKS The Basics of the Periodic TableĬreated in 1869, and modified several times since then, the periodic table of the elements developed by Russian chemist Dmitri Ivanovitch Mendeleev (1834-1907) provides a highly useful means of organizing the elements. The nonmetals form a loosely defined cross-family grouping, as do the metalloids. Families on the periodic table include, in addition to noble gases and halogens, the alkali metals, alkaline earth metals, transition metals, lanthanides, and actinides. Despite these apparent differences, common electron configurations identify the halogens as a family. Fluorine is a member of another family, the halogens, which have so many shared characteristics that they are grouped together, despite the fact that two are gases, two are solids, and one -bromine -is one of only two elements that appears at room temperature as a solid. All noble gases, for instance, tend to be highly nonreactive: only a few of them combine with other elements, and then only with fluorine, the most reactive of all substances. Some are wildly creative.The term "family" is used to describe elements that share certain characteristics -not only in terms of observable behavior, but also with regard to atomic structure. In a few cases, you'll need to understand a bit about the element to ''get'' the poem, but most of the verses are accessible. So far, about one-third of the elements boast hyperlinked poems penned by a variety of chemist-poets. If you have a favorite element and a literary bent, the Periodic Table of Poetry (n2o.com/elemental) awaits - emulsion recollected in tranquillity. James Holler, who share a belief that comic books spurred them to become chemists.Ī visitor can click on an element to see a comic strip panel featuring that element, like this excerpt from a 1967 ''Metamorpho'': ''My stinger injects a chemical cocktail of the world's most potent elemental poisons, from lead to arsenic to chlorine, krypton, selenium and strontium!'' Professor Selegue said that using the table to organize the site was a no-brainer: ''It's the chemist's alphabet.

One such effort is Chemical Comics (periodic.html), a labor of love by two University of Kentucky chemistry professors, John Selegue and F.

For children, the Chem4kids site (index.html) is a fine introduction, while the University of Kentucky's chemistry department invites know-it-alls to have a go at a table that is blank (periodicquiz.html).īut Periodic Table sites are not limited to the merely useful. Both provide an enormous amount of information about each element, easy searching and options for printing. Two excellent Web versions of the table can be found at Web Elements (and the Pictorial Periodic Table (chemlab.pc./ periodic/periodic.html).


 0 kommentar(er)
0 kommentar(er)
